Epigenetics is the study of the changes that occur on top of the DNA without changing the genome sequence, but still affect the expression of our genes and thereby shape our phenotype. This is the reason why even identical twins, who have almost the same genome sequence, are different from each other: due to their distintc epigenome.
Epigenetic traits, unlike the genome, are configured all along development. They are flexible and reversible. This is the reason why we can have so many distinct cells in our body, each with its own shape, size and molecular characteristics that determine their function. And that is also why scientist can reverse almost any somatic cell into a pluripotent cell (cells that are able to differentiate, or mature, into the three primary groups of cells that form a human being: ectoderm, endoderm and mesoderm); important for cell therapy.
Nevertheless, the epigenome is also heritable across generations. It is influenced by the dietary habits and life styles of our parents. However, they can be reversed by our own habits and pass the changes to our children.
The main epigenetic changes in an organism encompass DNA methylation and Histone modifications. In order to have a clearer idea, let us discuss them in detail.
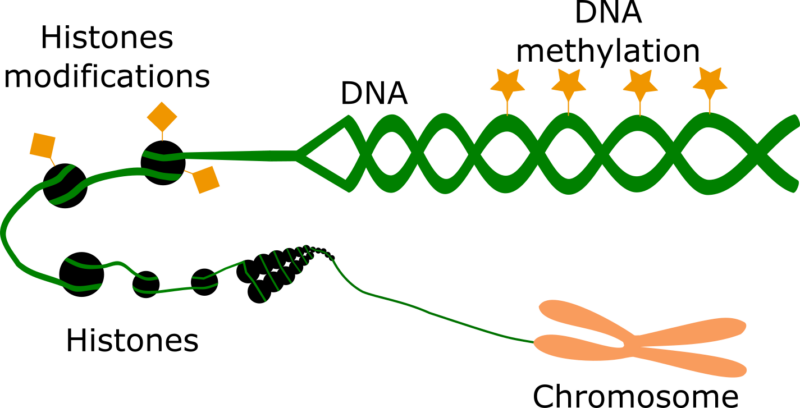
DNA methylation is basically the addition of a methyl group on top of certain regions of the DNA sequence. When DNA methylation occurs close to the sequence of a gene, it represses the expression of this, drastically. DNA methylation is essential for normal development. It is commonly erased during zygote formation, but is re-established in the embryo at around the time of implantation. Most DNA methylation plays important roles in genomic imprinting and X-chromosome inactivation, and when dysregulated, contributes to serious diseases like cancer.
Histone modifications entail a number of chemical changes that occur on top of, so called, histone proteins. These proteins served as reels for the long DNA to be packed inside our cells. The more packed a gene region is, the more difficult for it to express and define a phenotype. The most canonical histone modifications encompass histone acetylation and methylation. Apart from altering chromatin structure, these modifications determine the recruitment of other proteins to the DNA in order to activate or deactivate certain genes and even to repair damaged regions of the genome. Thus, histone modifications are relevant for the correct expression of genes and to avoid cells with abnormal genomic information. They are associated with a number of diseases and their state can be used to monitor the efficacy of a medical treatment.

