“What is cell therapy and what does it have to do with epigenetics?”, one might ask. Well, frankly speaking, cell therapy could be defined as treatment with cells. In fact, it is an approach by which cellular material or a biological product that has a proven therapeutic effect is derived from living cells, and is then injected or transplanted into a patient [1]. Cell therapy is a common application in immunotherapy, where it works on artificially stimulating the immune system (training it) to improve its recognition of a certain disease [1].
The advancement in cancer immunology accelerated the clinical applications of immunotherapy and, consequently, of cell therapy. Therefore, cell therapy has mainly been used in cancer treatment. One of the effective treatments is the Chimeric Antigen Receptor T-cell therapy (CAR-T) [2]. Shortly, CAR-T is based on the principle of extracting T-cells – which are important white blood cells of the immune system – from patients’ blood samples, reprogramming them using the CAR gene and infusing the modified T-cells back into the patients [2]. See figure below for more information.

The traditional approach of combining other immunotherapies with CAR-T therapy has been applied in the last years. The ultimate goal is to achieve a synergistic effect – that is, the interaction between two factors (in this case drugs or therapies) of related outcomes working together to achieve a greater outcome. Nevertheless, already-existing applications (e.g. CAR-T cells plus Immune Checkpoint Inhibition) showed promising results, until most patients failed to derive clinical benefit from it or have developed resistance to such a treatment [3,4,5].
Hence, one has to think outside the box and start including other therapeutic models together with cell therapy in order to maximize patients’ benefit and overcome resistance. This is where epigenetics comes into play! Defined in simple terms, epigenetics is the study of the changes that occur on top of our DNA without changing the genome sequence, but they still impact the expression of our genes.
Epigenetic therapeutics have been used in combination with classic chemotherapies, targeted therapies, other epigenetic agents, and immune checkpoint inhibitors to broaden response rates among patients with hematologic cancers and to extend the reach of such treatments to solid tumours. Even though in vitro studies usually demonstrate synergy when different therapies are combined with epigenetic drugs, clinical results tend to show the opposite. In fact, up until now, only one combination of l chemotherapy plus an epigenetic drug received accelerated approval from the American Food and Drug Administration (FDA) [6]. That treatment includes Panobinostat (histone deacetylase inhibitor), Bortezomib (proteasome inhibitor), and Dexamethasone (glucocorticoid medication).
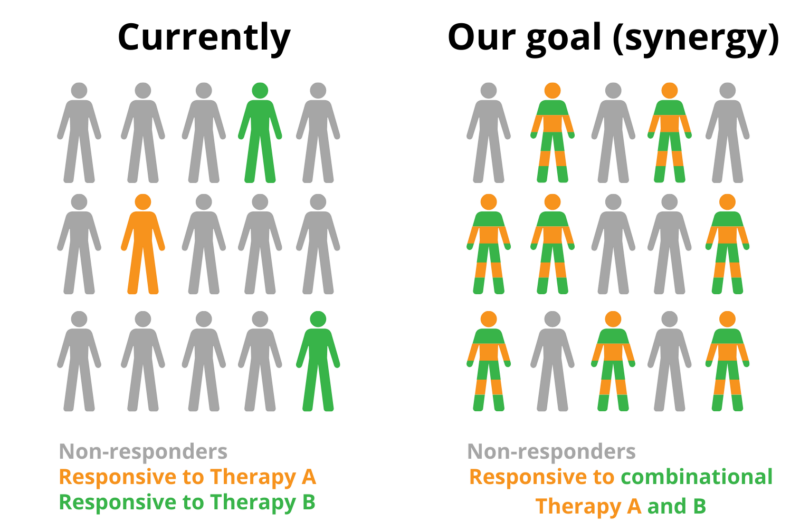
Over time, promisinging epigenetic candidates have been identified and shown to be synergistic. For example, in 1983, combinations of DNMT-HDAC inhibitors showed promising synergy [7]. However, despite extensive studies, there has been no solid evidence on the efficacy of epigenetic drugs on, for example, acute myeloid leukemia (AML) treatment [8]. As of now, numerous combinations with immune checkpoint inhibitors are being studied, one recent example is EZH2 inhibitors [9].
There is a lot that needs to be uncovered to reach the point of successfully combining epigenetic drugs with cell therapy. Through collaborative research, EpiQMAx aims to be at the forefront in discovering new epigenetic insights. Our hope is that such findings may eventually guide the development of agents, and combinations of agents that can be used in precision medicine.